Research and Innovations
Welcome to Innovative Solutions! Dive into the world of cutting-edge liposomal drug delivery systems.
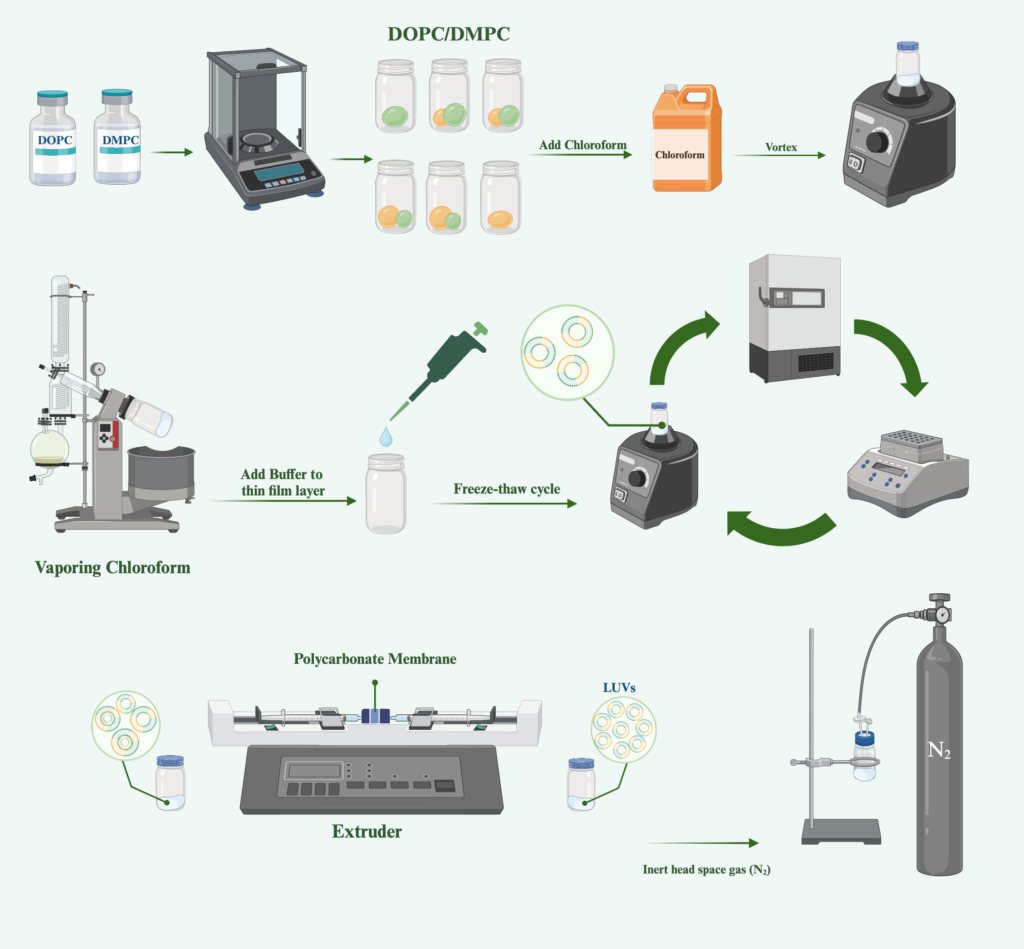
Liposomal Formulation Design
Parameters including phospholipid tail length and degree of unsaturation in the tails, contribute to the overall performance characteristics of liposomal formulations. Therefore, the choice of lipid for formulating liposomes is critical, as it directly influences the quality, safety, and performance of the final product. In this investigation, drug-free liposomes were synthesized utilizing phospholipids incorporating a blend of saturated and unsaturated long-chain fatty acids, characterized by carbon atom lengths of 14 and 18. The liposomal composition comprised two distinct lipid components: 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC).
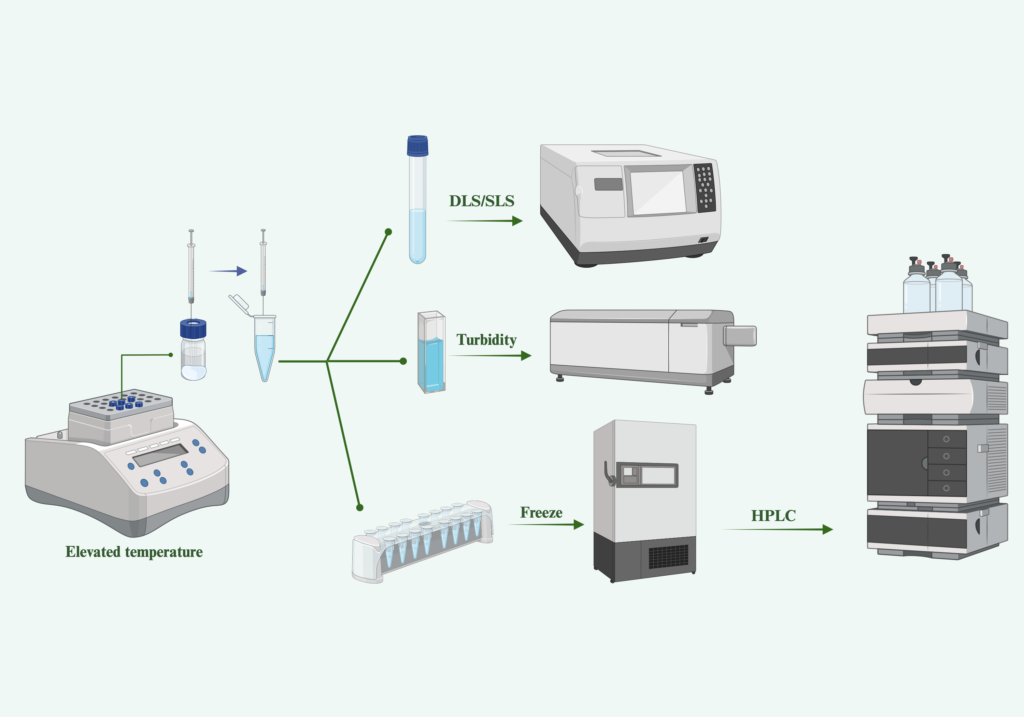
Stability Testing Solutions
The stability of liposomes as carriers for drugs is a critical aspect of their design and utilization. Various factors, including temperature fluctuations, pH levels, and the presence of degrading agents, can impact both the structure and functionality of liposomes. Consequently, the stability of functional liposomes must be thoroughly assessed, considering both chemical and physical changes. Various factors can influence the physical stability of liposomes, including their integrity, size distribution, and the presence of unsaturated fatty acid groups. The size of liposomes significantly impacts various in-vitro attributes of the nanocarrier, encompassing drug loading capacity, aggregation tendencies, and sedimentation rates. It is important to determine the lipid composition since lipid formulation has been designed to meet a specific need in drug delivery systems. Quantification of lipid components is a key attribute related to drug incorporation and release rate, pharmacokinetic properties, and stability. Normally, more than two lipid compositions with a specific ratio are chosen to develop liposome formulations; examination of lipid species and amounts in the final preparation becomes a crucial step to ensure the lipid formulation is developed as needed.
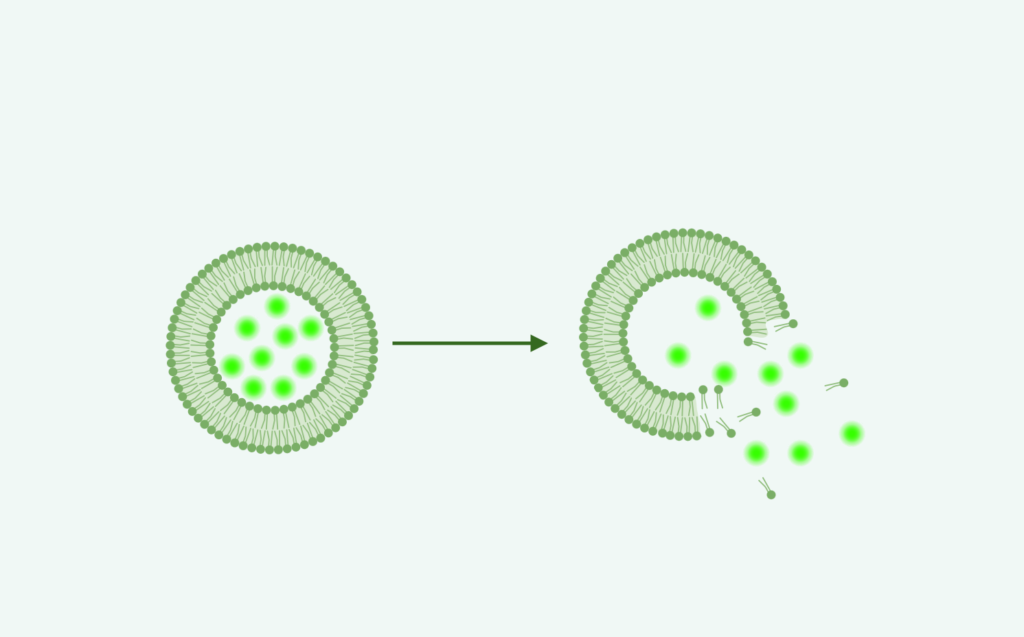
Drug Release Studies
In Progress
Results
In Progress
